In the field of medical electronics, every PCB and PCBA carries the weight of life and the health of patients. Even a minor defect can lead to catastrophic consequences. This makes the procurement of medical device circuit boards far more than a cost consideration—it is a rigorous challenge of regulatory compliance and ultimate quality. We understand that electronic engineers focus on material and process details, hardware project managers emphasize project timelines and risk management, and procurement specialists evaluate supplier qualifications and cost-effectiveness. So, how can you meet these multidimensional requirements while keeping your medical products at the forefront of industry standards?
1. Regulatory Foundations for Medical Device PCB/PCBA: ISO 13485 and IPC Class III
In medical electronics manufacturing, meeting strict international regulatory standards is paramount. ISO 13485 is recognized as the highest standard in the medical device industry, ensuring full lifecycle quality control—from design and development to production, installation, and service. Simultaneously, IPC J-STD-001 and IPC-A-610 Class III serve as the benchmark for manufacturing and accepting medical-grade PCBs and PCBAs. These standards represent the highest requirements for reliability, long service life, and operation in demanding environments. Achieving these standards not only ensures compliance but also guarantees stable and safe product performance.
2. Material Selection and Traceability: The Source of Reliability
Material selection is crucial for medical PCBs, impacting not only electrical performance but also stability and durability in specific medical environments. In some applications, engineers also consider biocompatibility—even though PCBs generally do not directly contact the human body, they must ensure that materials are pure and the production environment meets strict standards. Manufacturers commonly use high-Tg (glass transition temperature) materials to maintain dimensional stability and electrical performance under high temperatures.
Every batch of raw materials is fully traceable, with batch numbers, country-of-origin certificates, and quality inspection reports. Detailed material management is the first line of defense against potential risks.
Technical Term: High Tg — Refers to the temperature at which PCB substrate material transitions from a rigid to a softened state. High-Tg boards maintain better dimensional stability under high-temperature conditions, making them suitable for high-reliability applications.
| Performance Metric | Standard FR-4 | High-Tg FR-4 | Rogers Material | Ceramic Substrate | Flexible Substrate (Polyimide/PI) |
|---|---|---|---|---|---|
| Glass Transition Temperature (Tg) | ~130-140°C | ≥170°C | Variable, usually high | >800°C | ~260°C |
| Dielectric Constant (Dk) | ~4.5 | ~4.0-4.8 | Low and stable (2.3-10.2) | Variable | ~3.4 |
| Dielectric Loss (Df) | ~0.016 | Lower | Extremely low (<0.005) | Low | Low |
| Thermal Conductivity (W/m·K) | Low (~0.3-0.5) | Slightly higher (~0.4) | Moderate (0.26-0.70) | Very high (24-180) | Low |
| Compliance | UL 94V-0, RoHS/REACH | UL 94V-0, RoHS/REACH | UL 94V-0, RoHS/REACH | RoHS | RoHS/REACH |
| Medical Application Advantages | Cost-effective, suitable for general medical electronics | High-temperature resistance, suitable for sterilizable or high-power devices | Excellent high-frequency performance, suitable for MRI and RF equipment | Superior thermal performance and biocompatibility, ideal for implants, CT, X-ray | Lightweight, flexible, suitable for wearable monitors, implants, miniaturized instruments |
| Limitations | Performance degrades at high frequencies | Higher cost than standard FR-4 | Very high cost | Difficult to process, expensive | Limited load-bearing capacity, higher cost |
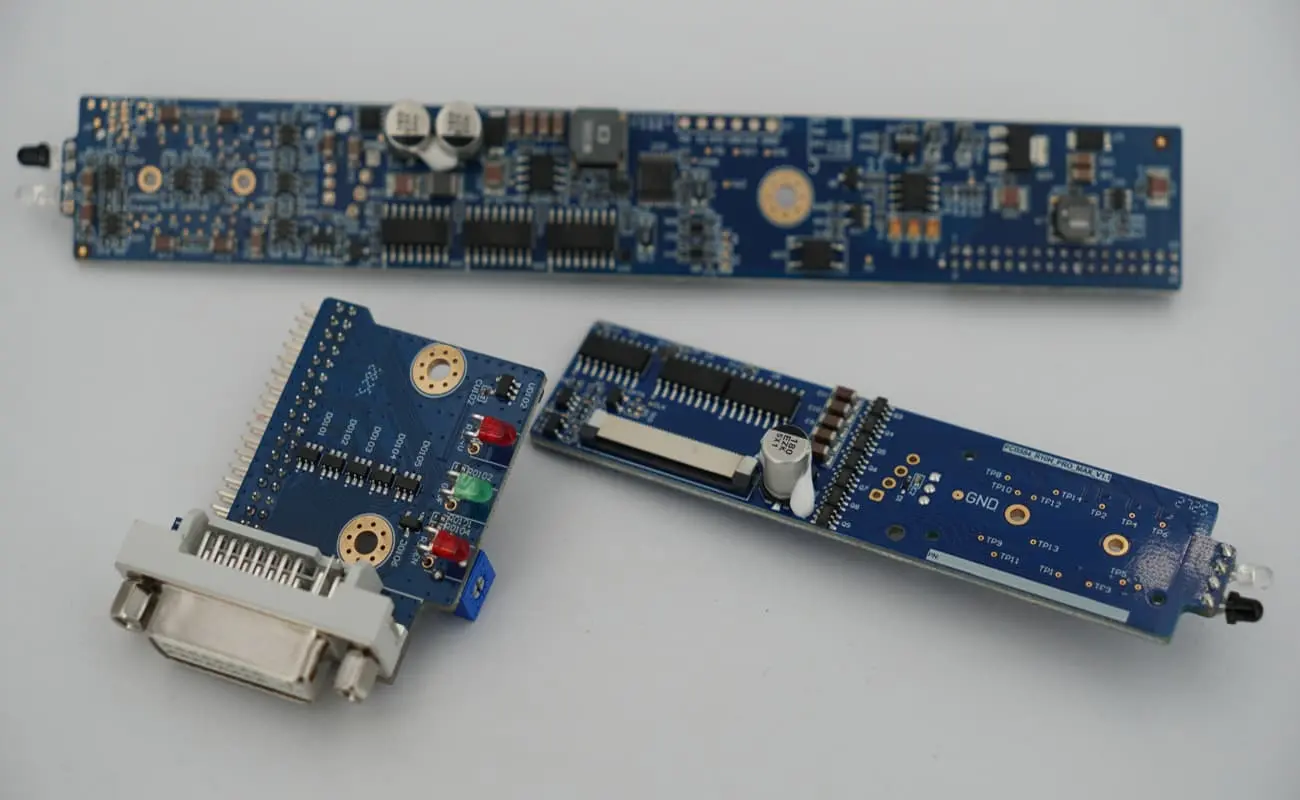
3. Precision Manufacturing: Turning Design into Excellence
Medical PCB manufacturing demands extreme precision. Any deviation can cause performance degradation or failure. From precise trace design (such as impedance control), strict cleanliness standards, and cleanroom production, to accurate component placement (e.g., BGA soldering) and lead-free soldering, every step must be flawless.
Fully Hong implements advanced automated production lines and strict ESD (electrostatic discharge) control to ensure process stability and consistency. DFM (Design for Manufacturability) and DFA (Design for Assembly) analyses are conducted early in collaboration with client engineers to optimize designs and effectively reduce manufacturing risks and costs.
Technical Terms:
-
Impedance Control: Designing PCB trace width, spacing, and dielectric thickness to ensure proper impedance matching, avoiding signal reflection and distortion.
-
ESD Control: Using anti-static materials, grounding measures, and humidity control to prevent static damage to sensitive components.
4. Comprehensive Quality Control and Testing: Unmatched Assurance
Quality is not a result—it is a process embedded throughout manufacturing. For medical PCBA, multi-level inspections are conducted: AOI (Automated Optical Inspection) for component placement and soldering defects, X-Ray inspection for hidden BGA and CSP solder joints, and FCT (Functional Circuit Testing) to ensure compliance with design specifications.
Environmental reliability tests, including high/low temperature cycling and vibration tests, simulate extreme conditions during product use. All test data are recorded and archived to ensure traceability for every production batch.
Technical Terms:
-
AOI (Automated Optical Inspection): Uses optical principles to detect PCB defects such as shorts, opens, misalignment, missing solder, or insufficient solder.
-
FCT (Functional Circuit Testing): Simulates real-world operation to verify that the product meets design specifications and performance requirements.
5. Supply Chain Management and Project Timeline Optimization: Efficiency and Stability
In medical device development and manufacturing, project timelines and cost control are key concerns for hardware project managers and procurement specialists. Selecting a supplier with strong supply chain management and industry experience is critical.
Fully Hong has established long-term partnerships with leading global component suppliers, ensuring timely supply and reliable quality. Rapid prototyping and scalable production capabilities help shorten product time-to-market. Lean manufacturing and strict cost control ensure competitive pricing while maintaining medical-grade quality.
Why Choose Fully Hong? Your Strategic Partner in Medical Electronics
In medical PCB and PCBA, Fully Hong is not just a supplier—it is a trusted strategic partner.
-
Deep Regulatory Expertise: We strictly follow ISO 13485 and IPC Class III standards, ensuring your products comply with global medical regulations.
-
Comprehensive Quality Assurance: From DFM and material traceability to multi-level precision testing, we maintain near-zero defect rates.
-
Advanced Manufacturing and Technical Expertise: Our modern production lines and experienced engineers handle even the most complex medical PCB and PCBA designs.
-
Efficient Supply Chain and Cost Optimization: Strong global supply networks and flexible production capabilities ensure on-time delivery and cost-effective solutions.
Fully Hong helps you achieve safe, reliable, and regulatory-compliant medical electronics, ensuring your products lead in quality, innovation, and market success.






Leave a reply